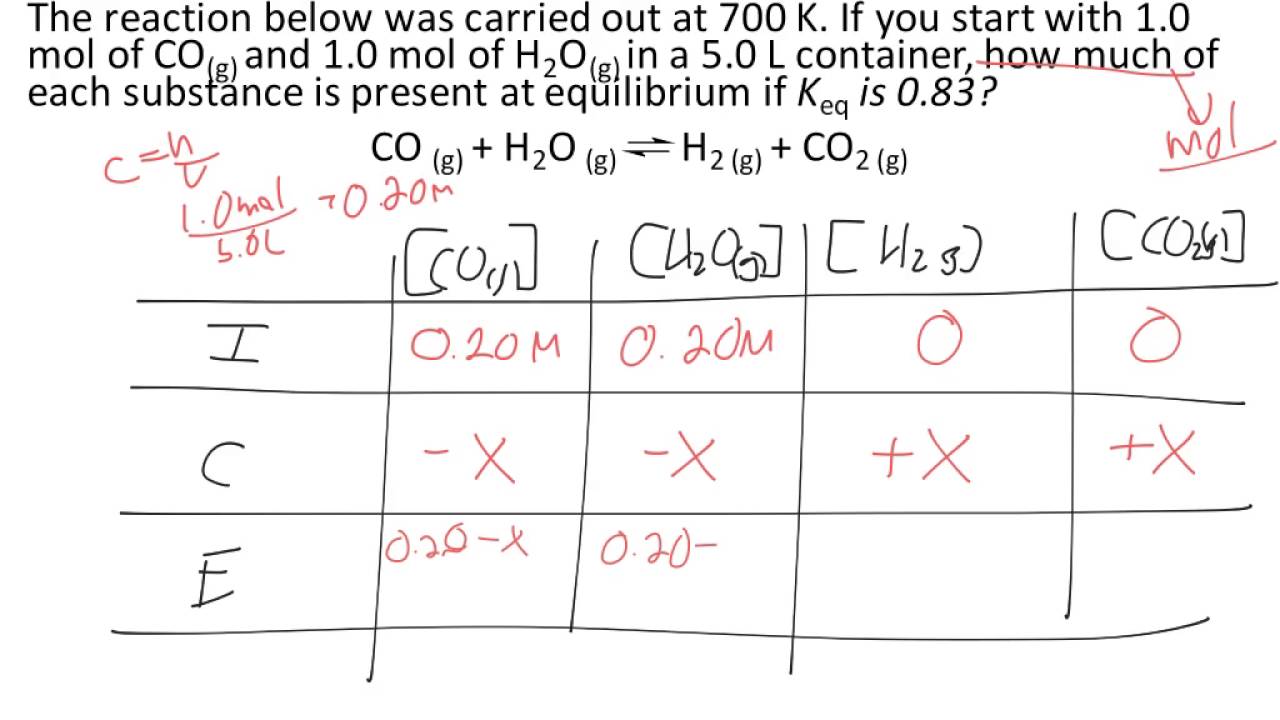
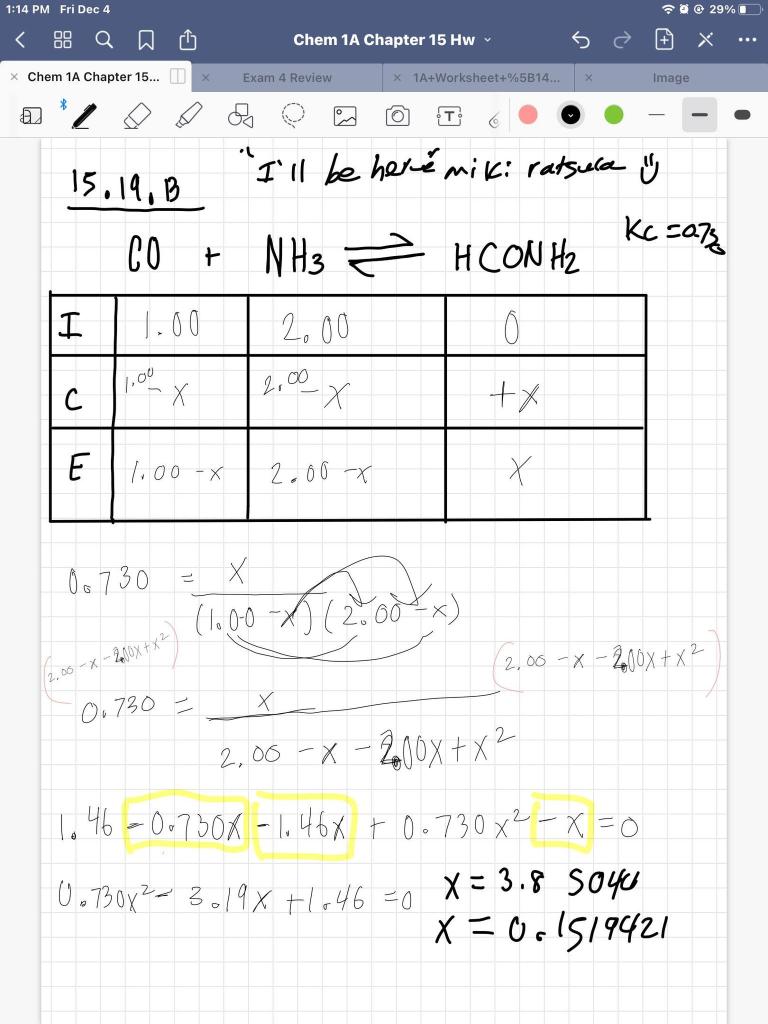
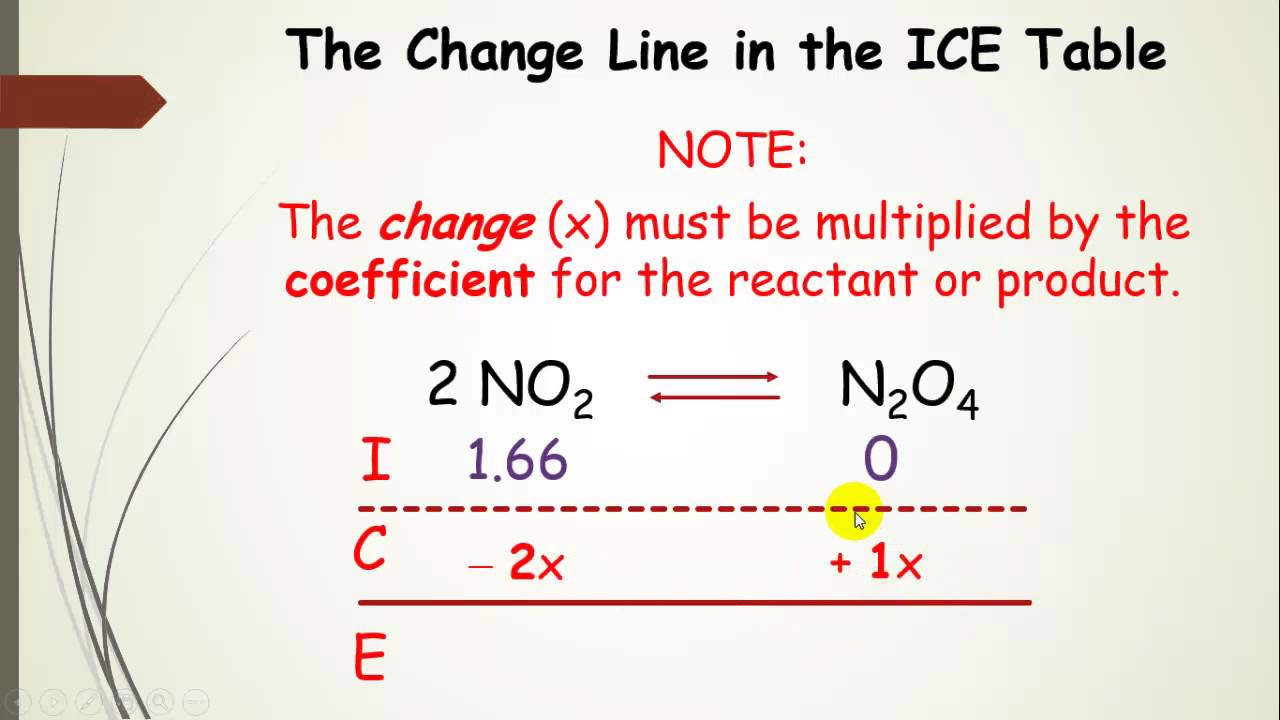
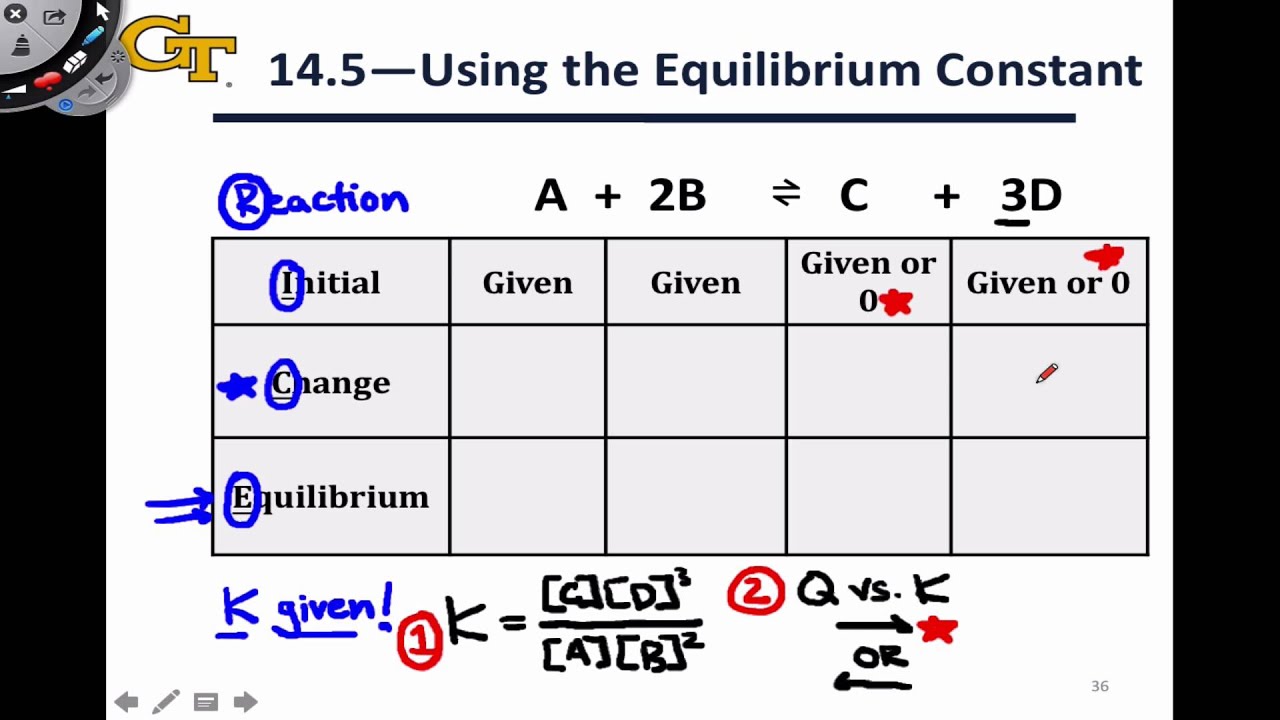
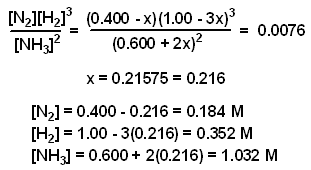ICE Table Practice Problems - Initial Concentration, Equilibrium Concentration, Kc (Part 1) - YouTube
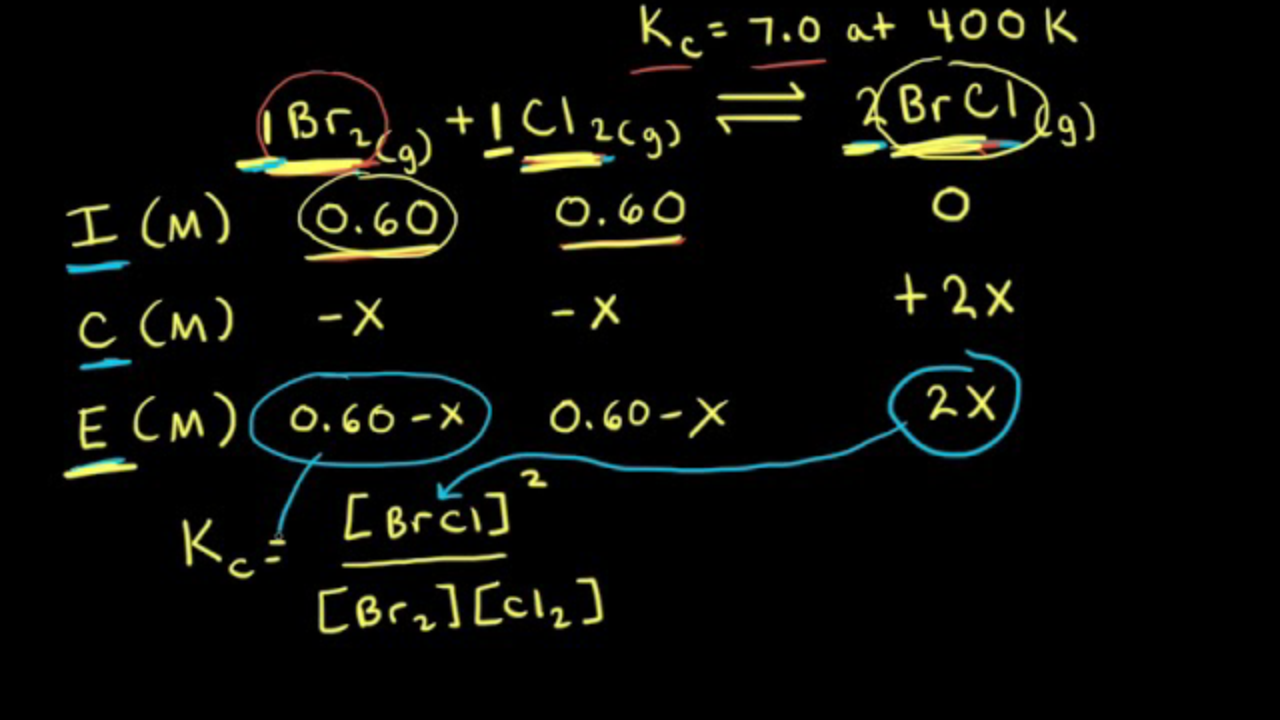
Calculating equilibrium concentrations from initial concentrations and the equilibrium constant (worked example) (video) | Khan Academy
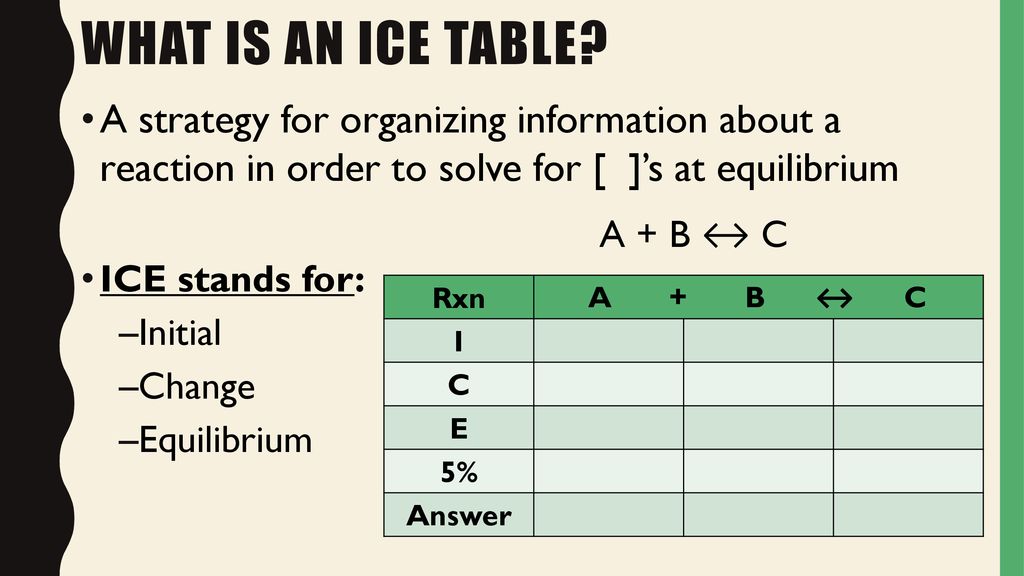
N45 ICE Tables Target: I can find the concentration of reactants and products at equilibrium using an ICE table. - ppt download
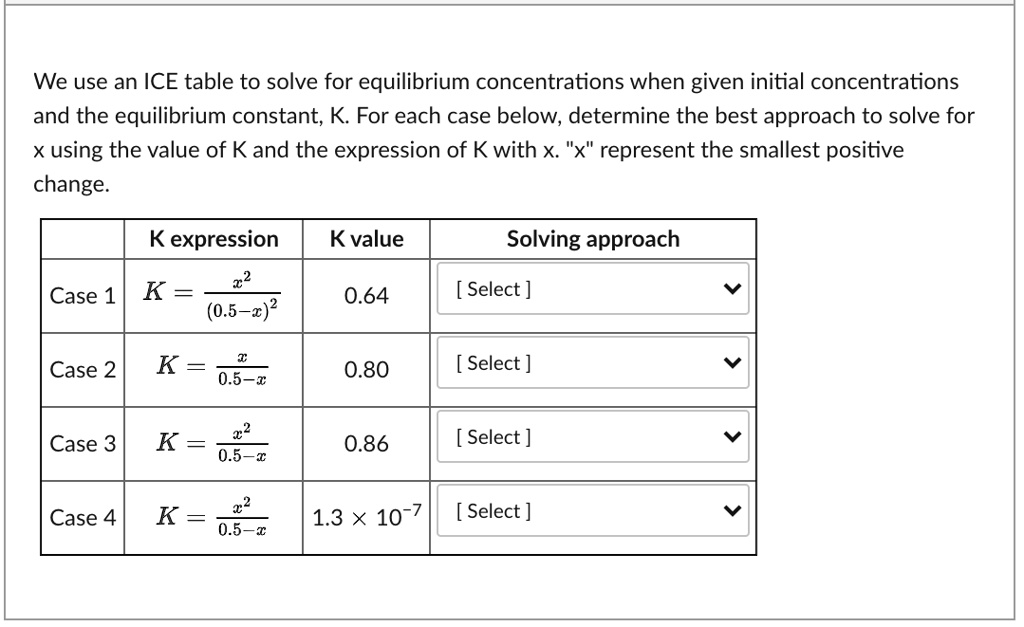
SOLVED: We use an ICE table to solve for equilibrium concentrations when given initial concentrations and the equilibrium constant; K For each case below, determine the best approach to solve for using

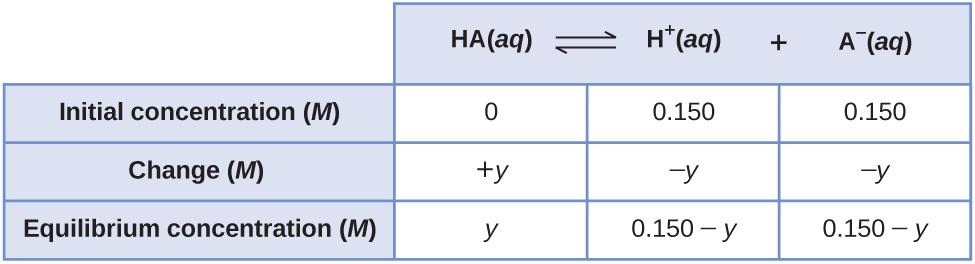
How do use the ICE table to find the stoichiometric concentration of the two important species in the buffer? | Homework.Study.com

SOLVED: Setting up an ICE table to calculate Kc: Iodine molecules react reversibly with iodide ions to produce triiodide ions. I2 (aq) + 2I- (aq) ⇌ I3- (aq) If a solution with
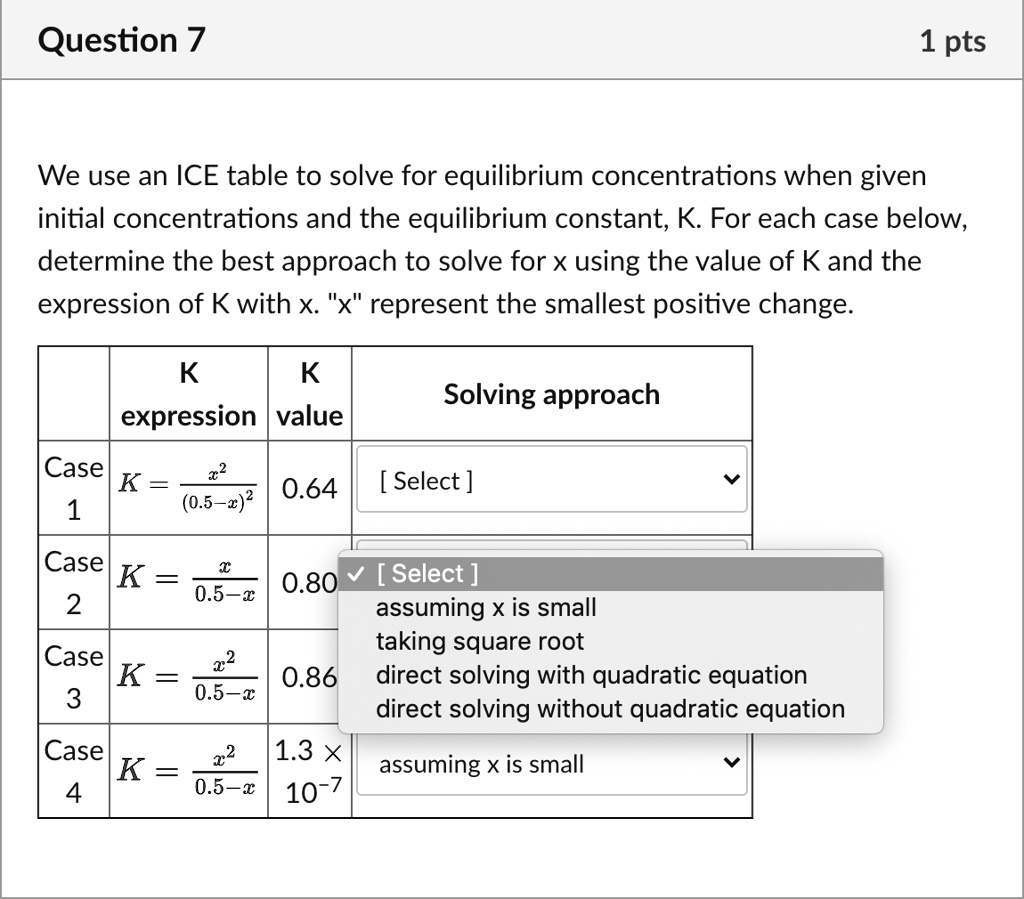
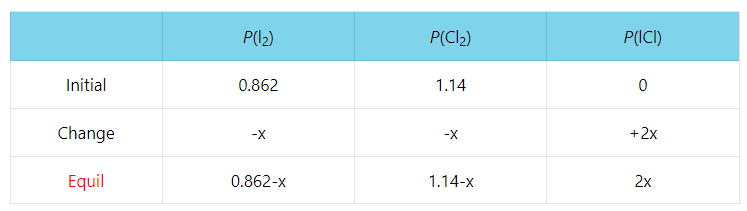
SOLVED: We use an ICE table to solve for equilibrium concentrations when given initial concentrations and the equilibrium constant, K. For each case below, determine the best approach to solve for X