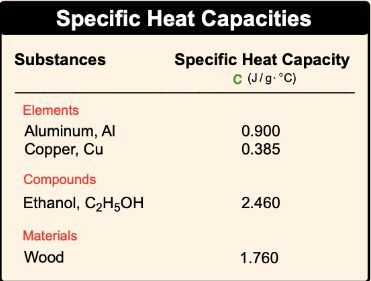
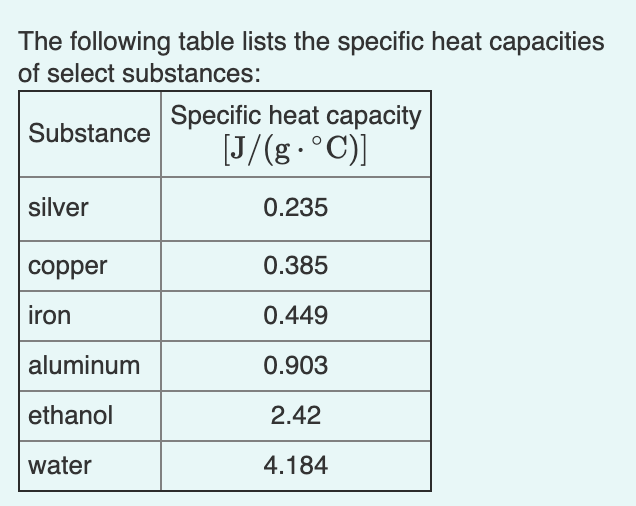
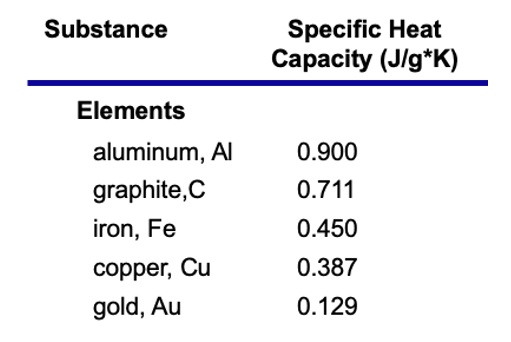
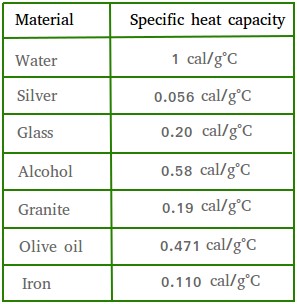
Calculate the energy required to heat 790.0g of iron from −2.6°C to 14.9°C. Assume the specific heat - brainly.com

The Heat Capacity and Thermodynamic Properties of the Iron Oxides and Their Relation to the Mineral Core of the Iron Storage Protein Ferritin | Semantic Scholar

Derived mean values of the specific heat of pure iron in comparison... | Download Scientific Diagram
Molten iron is extremely hot, averaging about 1,500 C. The specific heat of iron is 0.46 J/gC. How much heat is released to the atmosphere when 1 kg molten iron cools to

Comparison between the calculated and experimental heat capacity of... | Download Scientific Diagram


![Molar heat capacity of iron. Symbols indicate measurements from [10]. | Download Scientific Diagram Molar heat capacity of iron. Symbols indicate measurements from [10]. | Download Scientific Diagram](https://www.researchgate.net/publication/332219094/figure/fig1/AS:744154090987520@1554431569138/Molar-heat-capacity-of-iron-Symbols-indicate-measurements-from-10.png)